Q: What size aquarium heater do I need?
Q: Do I need an aquarium chiller? What size aquarium chiller do I need?
A:If you want to move past the general “rules of thumb” thrown around (even here on beananimal.com) you can use our favorite subject (physics) to get a better idea of how much heat your aquarium will give up to (ro gain from) the surrounding room. If we know how much heat the aquarium will lose to the room, we know how much heat we must add to maintain a constant target temperature in the aquarium. To answer the question, we need answer a few more questions to help us work through the steps:
1) Calculate how much heat the tank will lose to the room during the time period in question. We will need to look at both conduction (heat through the glass) and evaporation (heat lost due to the phase change from water to vapor).
2) calculate how much heat is added to the tank during the time in question. Things like lights and pumps add heat back into the system.
3) Determine the difference between the heat lost and the heat added so that we can properly size a heater.
Lets get started…
Q: How much heat is transferred through the aquarium glass into the room?
A: We can use Fourier’s Law (the law of heat conduction) to examine the conductive heat transfer from your fish tank to the surrounding environment. So that we do not need to duct tape our heads together to keep the brain matter from exploding, we can skip the definition and assume that Joseph Fourier knew what he was talking about back in 1801. The Fourier Conduction formula can be used to give us an idea of how fast heat will travel through a solid, in our case from the aquarium water, through the glass panel and into the air in the room.
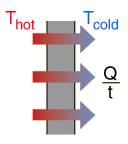
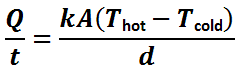
| Q/t | = | Heat Conduction in BTU/hour |
| k | = | Thermal Conductivity |
| A | = | Area in square feet |
| d | = | Thickness in feet (inches ÷12) |
| T | = | Temperature in degrees Fahrenheit |
We can use the equation to determine how much heat will flow through the glass panels of the aquarium. We will assume that we know the daily evaporation of the aquarium through the open top and therefore we will ignore convective heat loses (minimal in comparison) through the open top of the aquarium. We will also treat the bottom of the tank the same as the sides, even though it is covered by a mass of sand and partially insulated by the stand.
Example: As an example we will look at a typical 75 gallon aquarium with dimensions of 48″ x 18″ x 20″ constructed with 3/8″ glass and a target operating temperature of 80° F with an ambient room temperature of 72° F.
Area:
front 48″ × 20″ = 960 sq. inches
Back 48″ × 20″ = 960 sq. inches
Left 18″ × 20″ = 360 sq. inches
Right 18″ × 20″ = 360 sq. inches
Bottom 48″ × 18″ = 864 sq. inches
_________________________________
So:
960 + 960 + 360 + 360 + 864= 3505 sq. inches (simply add up the total area of all of the panels)
Then:
3505 sq inches ÷ 144 = 24.340 sq. feet (there are 144 square inches in a square foot)
d: (thickness)
3/8″ = 0.375 inches
0.375 ÷ 12 = 0.0313 feet (convert inches to feet)
k: (thermal conductivity)
We can find the thermal conductivity of glass by looking it up in a table. The thermal conductivity of glass can vary depending on the types of minerals found in it. For our purposes we will use k = 0.578 BTU/hr ft°F (if you aquarium is made of acrylic, then your thermal conductivity constant will be k = 0.12 BTU/hr ft°F and likewise for wood it will be k = 0.07 BTU/hr ft°F)
Once we plug in the numbers our equation (for the glass tank in our example) will look like:
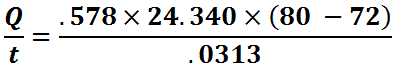
Doing the math we get a result of 3595.79 BTU/hour of heat transfer. We still need more information to properly size our heater… Read On!
Q: How many Watts are in a BTU?
A: In reality the question should be “How many Watt/hours are in a BTU?“. A Watt is a measure of energy used per unit time (also called POWER).
1 BTU = 0.293 Watt/hours
1 Watt/hour = 3.412 BTU
With that handy information we can figure out how many Watts it will take to keep our aquarium at the target temperature. But wait! We have a few more things to consider…
Q: How many BTUs does the aquarium lose per gallon of saltwater evaporated?
A: In the spirit of keeping things simple, we will forgo the duct tape around the head and skip the scientific definitions in favor of some basic facts. At atmospheric pressure, the Latent Heat of Vaporization (evaporation) of pure water (at sea level) is about 970.4 BTU/lb. Seawater (the stuff in your reef tank) has a slightly lower Latent Heat, lets say 960 BTU/lb. Seawater weighs about 8.5 pounds per gallon. So it takes somewhere in the neighborhood of 8160 BTU to evaporate 1 gallon of seawater. But how does that help us? Read on…
Q: How many BTUs does it take to raise or lower the water temperature in my aquarium?
A: It takes 1 BTU to raise (or lower) 1 Pound of water 1° Fahrenheit. That is, the specific heat of pure water is 1.0. Pure water weighs about 8.34 pounds per gallon. So it takes about 8.34 BTU to raise 1 pound of water 1° F. That is likely close enough for most of us.
For those of you wish to be a little more accurate:
The specific heat of saltwater (seawater) is about 0.952 and seawater weighs about 8.5 pounds per gallon. So therefore it takes about 8.1 BTU to raise (or lower) 1 gallon of seawater 1° F.
Q: How much heat does my submersible pump add to the aquarium?
A:For our purposes, almost 100% of the energy consumed by a submersible pump (mag-drive, powerhead, etc.) is converted to heat in the tank! The simple fact is that if you were to take a heater that consumed exactly 100W and a powerhead that consumed exactly 100W and placed them each in a 5 gallon bucket with a lid, both buckets would reach the same temperature in the same amount of time! Why? Much of the energy consumed by the pump is directly shed as heat into the water. What is left does work and that work (moving water) causes friction (water moving against the objects and walls of the tank), most of which is converted to heat in the tank. When attempting to calculate the heat load on your tank, you should add up the total wattage of all submersible pumps in the system!
Q: How much heat does my external pump add to the aquarium?
A: This is not as cut and dry as a submersible pump. Different styles of external pumps contribute different amounts of heat into the system. Some external pumps are cooled by the water flowing through them (most mag-drive pumps). Most Jet style centrifugal pumps are fairly well isolated from the system and may only shed 5%-25% of their heat into the aquarium (remember they move water and create friction, no matter what). Is there a rule of thumb? Not that I know of! I would assume maybe 80% heat transfer for external mag-drive style pumps and 15%-25% heat transfer for other external pumps, especially if they are located in a closed stand!
Q: How much heat do my lights add to the aquarium?
A:This also is a complex question and depends on many factors. For our purposes we can simply use the radiant energy of the lighting family as a reasonable estimate of the heat transfer into the aquarium. If you look at the chart below, you will see that both Metal Halide and Fluorescent lighting technologies both have similar radiant energy figures. LED lighting has a much lower radiant energy and therefore contributes less heat directly to the water. It should be noted that no matter what lighting technology is used, they ALL contribute exactly the same amount of overall heat (per Watt) into the room! Please examine the following table (available from the United States Department of Energy).
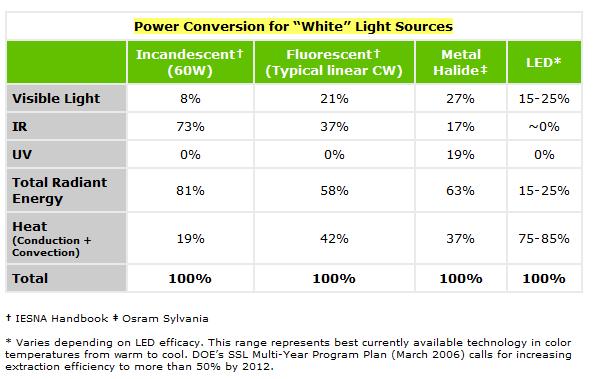
In other words, if you have 250W worth of Metal Halide lighting above your aquarium, you can somewhat accurately conclude that 63% (about 157 Watts) of that energy will end up as heat radiated into the tank. Note that a portion of that 157 Watts of energy will never make it into the tank and some of it will escape the tank before it is turned to heat in the water. At the same time, a portion of the leftever 37% that is shed from the fixture via conduction and convection (trapped in the hood and/or room) will contribute to heat in the tank. If the aquarium does not have a hood, then you can assume that somewhat less heat will be transferred to the aquarium water. If the aquarium has a poorly ventilated hood then you can assume that more of the total energy will end up as heat in the aquarium water. There are simply too many variables to attempt to actually make an accurate calculation further than the riugh estimated explained above.
Q: Can you help me put it all together?
A:You know how to calculate how much heat your aquarium will lose to the room. You know how many Watt/hours it takes to produce a BTU of heat. You know how to, based on the volume of water in your aquarium, how many BTUs it takes to raise or lower the temperature of the saltwater in your aquarium. You know how much water your aquarium evaporates over a 24 hour period. It is a simple task of putting the numbers together to determine how big of a heater or chiller you may need.

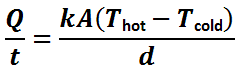


Add comment